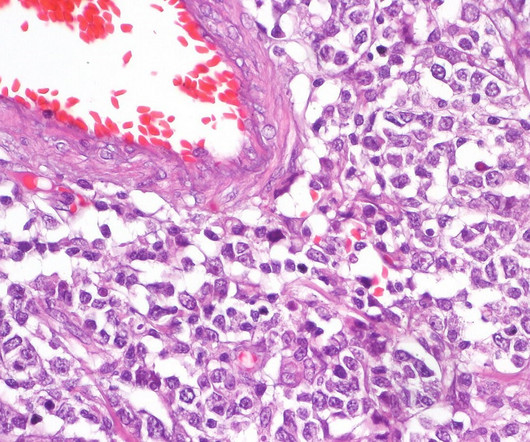
Oncology dominates CRISPR landscape: GlobalData
Express Pharma
MARCH 6, 2024
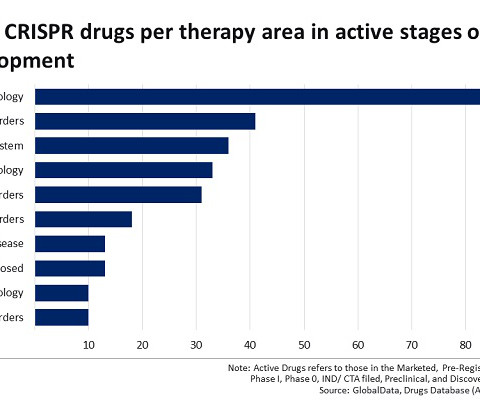
The Medicines and Healthcare products Regulatory Agency (MHRA) approved Vertex ’s exagamglogene autotemcel (Casgevy) in late 2023. Despite this approval, the CRISPR landscape is dominated by oncology, which accounts for 30 per cent of all active CRISPR drugs.

































Let's personalize your content